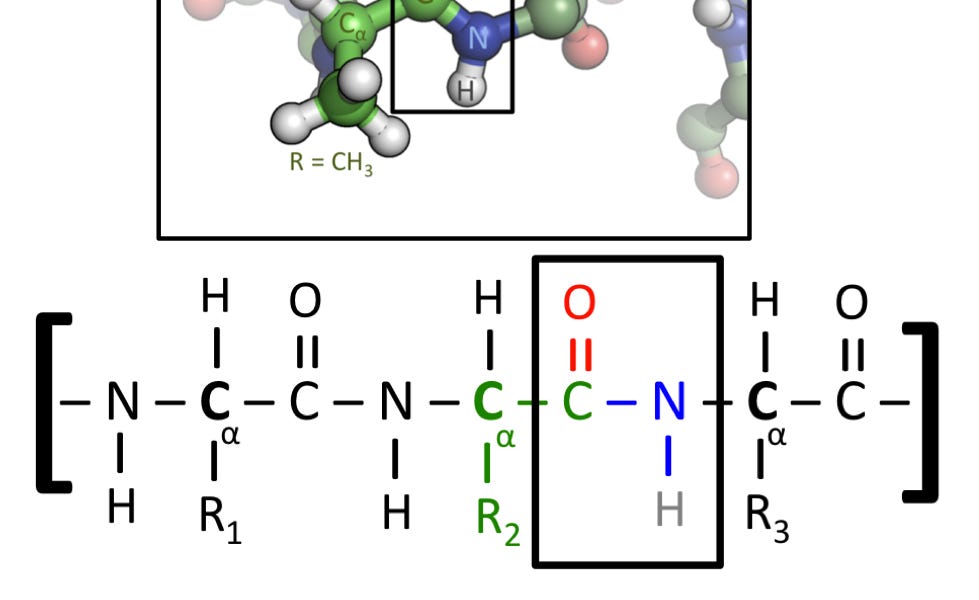
This is my article on one of the dumbest and most obviously false claims Yudkowsky has ever made, about biology not using covalent bonds.
I’ve been looking through the replies in the crossposts. It illustrates your gullibility filter point completely.
Also, if you need validation, you are a fantastic science communicator. You take research that isn’t accessible to me and present it in a form I can understand. You also provide enough disclaimers about gaps in your knowledge when you might be glossing over details while providing me with inroads to research those details themselves. You dispel fear and confusion stemming from the unknown while inspiring me to discover more.
Yud doesn’t do any of those things and often does the opposite, but let’s forget about him for a moment. Good article!
Thanks! I strive for accuracy, clarity, humility, and good faith. Aka, everything I learned not to do from reading the sequences.
Organic chemist there
bucket o' nitpicking incoming
I think that chemistry 101 classification of bonds is a tad useless here. Instead, you can go from first principles: there are things that happen when atomic orbitals overlap (covalent bonds, metallic and such), there are interactions that are mostly electrostatic in nature (ionic, dipole-dipole, quadrupole-quadrupole - important biologically as pi-stacking, also ion-quadrupole etc) and there are things that are a result of exchange interaction (van der Waals and steric repulsion). Hydrogen bonds would be a mix of dipole-dipole and van der Waals interaction. You don’t have to transfer electrons in order to have ionic interaction, most of the time in biologically relevant situations it’s proton transfer, or charges just were there previously. Hydrophobic interactions are almost entirely a solvent effect and aren’t a bond strictly speaking
In water, i’m pretty sure that proteins are mostly held by hydrogen bonds and hydrophobic interactions. EY is correct in that some proteins hold shape by mostly noncovalent interactions, but these are mostly hydrogen, ionic, hydrophobic interactions and the proteins that actually provide mechanical strength run in continuous covalent strands through entire length of them anyway (collagen, keratin). I don’t think that counting bonds and saying that something is 90% bound covalently is a meaningful metric, because long series of hydrogen bonds or even vdW forces (in things like UHMWPE fibers) can be stronger than single covalently bound strand, ie if you tried to pull out a single strand of kevlar or collagen from bulk material, above certain length you won’t pull it apart, you’d just break it because collective energy of hydrogen bonds will be greater than single covalent bond holding it together, that’s why these fibers are strong in the first place
There is another kind of flexibility that you haven’t mentioned: proteins are made out of single covalently bound strand, yes, but these aren’t straight C-C chains. Making and especially breaking C-C bonds in controlled way is hard, proteins can be just hydrolyzed at amide bonds. If protein breaks in some way, and in real world everything breaks, it can be recycled into aminoacids (+ any cofactors etc) and then put back in a pretty straightforward way; you can’t do this with diamondoids, when it breaks, it breaks hard, and you’re done unless you’re picking everything apart atom by atom which would be much harder and more energy intensive. As it happens you can buy bulk adamantane, but it’s just made in conditions where C-C bonds are weak (high temperature) and it’s preferentially formed because it’s most stable thermodynamically among its isomers (that are starting materials). Conversely, if you use weaker bonds, you can make pieces conform to some template, or to each other without breaking everything at once - this is basis of dynamic combinatorial chemistry. There’s also entire field of self-healing materials that is based almost entirely on these either noncovalent or reversible covalent bonds
As part of the process, parts of the enzyme actually shift and mold their structures around the incoming molecules in order to better catalyse reactions. I’m not sure how easily you could replicate this using stiff strictly covalent structures.
You actually don’t have to do that, and there are some small organocatalysts that are entirely covalently bonded and do the same job. However you can’t make them from from aminoacids, these don’t have secondary structure (too small) and are generally less active. The bare minimum is to provide a receptor for transition state, and you can make it work without drastic changes in conformation. You could make your catalyst as stiff as you like, and it’ll even make activity higher - but only if none of these stiff parts interfere with binding of substrates, and your options are limited. It’s often better to leave some wiggle room. Short peptides aren’t really stiff enough in ways that matter there and instead it’s secondary and tertiary structure that puts important bits in the right place
Feel free to pick my brain any time you like
Hey, thanks so much for looking through it! If you’re alright with messaging me your email or something, I might consult you on some more related things.
With your permission, I’m tempted to edit this response into the original post, it’s really good. Have you looked over Yudkowsky’s word salad in the EA forum thread? Would be interested in getting your thoughts on that as well.
my DMs are open, but lemmy’s DMs seem to be janky, matrix should be more reliable
I’m tempted to edit this response into the original post
no issues with that
Yudkowsky’s word salad
i’ll have a closer look tomorrow, for now i’d just say that that steel chain protein analogy is okay, however if you wanted to convey directionality of hydrogen bonds, then every link is magnetized, and really these links are not welded shut, but instead bolted, so you can disassemble them and put them together again with some effort. continuing this analogy, diamondoids would be elaborate welded assembly of stiff H-beams or something like that
i see that EY tries to “get” materials science from first principles, in true aristotelian fashion, never reading first year BSc level chemistry textbook, fails badly and can’t even comprehend that he can be wrong. in other words, another tuesday
Have you looked over Yudkowsky’s word salad in the EA forum thread? Would be interested in getting your thoughts on that as well.
update 2: i’m not doing that, he sounds like a straight up cultist in this one
update 3: unfortunately i had some time to look back at this, and the longer i thought about that “bacteria but stiffer” thing the more aggressively stupider entire thing becomes. get yourself a mean drink like i did, because it’s long. for your sneering pleasure (and if you want to use it, just rephase relevant bits it’ll be shorter):
here i'm talking out of my ass about psychology, so don't listen to me because i'm not a psychologist
Zeroth of all, let’s take a few steps back and look at the bigger picture. University allows you to learn, teaches you how to learn, but here one underappreciated thing is that it lets you fail non-catastrophically. Let’s say you know some things, and you arrive at a hypothesis. When you are in some professional environment dedicated to learning, you might be - should be - incentivized to test it: look up some literature, design an experiment, or just ask someone who actually knows. When you are right, you don’t really learn anything new; but when you’re wrong, you learn limitations of your method/approach/reasoning, how to fix them, new things etc but more importantly you learn some humility and nuance, and you learn to answer “it depends”. This of course doesn’t have to happen at university, but I suspect it’s pretty good at it. When you don’t have that, when you never are proven wrong, you might end up sniffing your own farts so hard that you refuse all criticism in the future, never admit that your ideas could be possibly faulty and you become EY. I know that because I’ve come too close for comfort to that path however at the uni some half year in where i had to do actual lab work cured me of it irreversibly when reality proved me wrong. I’ve also seen one case of exactly that on /r/chemistry, in that case there also was a complete lack of self-awareness about their own limitations, not really unlike of EY’s in principle, but he had more time to learn it. I can elaborate in DMs if anyone is so inclined
Everything breaks 1
For reasons that might be related to the above all rat’s nanomachines are perfect, never malformed, work flawlessly, fibers are infinite and without defects etc etc. Bitch, in real life everything breaks all the time, and yet reality and real things work. To illustrate this consider single kevlar fiber.
When taken alone, single molecular strand of kevlar is not significantly stronger than polyester, polyethylene, or PVC. However we don’t really use kevlar this way, and what makes kevlar kevlar is how it interacts with itself between strands. Every unit makes 4 rather strong hydrogen bonds perpendicular to strand direction, and perpendicular to both there are 4 quadrupole interactions between aromatic units. This all arranges single strands into sheets and sheets are then stacked parallel to each other. Now, let’s try to pull a single strand out of bulk material. When strands are short, it’s the hydrogen bonds and quadrupole interactions that break - strands just slip off and separate. However, above certain length, it’s easier to break single strand than to pull it out.
That length turns out to be quite small. Going for a ballpark figure, benzene C-H bond has dissociation energy of some 113 kcal/mol, while (quite similar) beta-sheet hydrogen bonds have some 4-9kcal/mol, depending on who you ask and how you measure. That works out to some 4 to 7 fully hydrogen-bonded diamine-diamide units are already held in place with more force by hydrogen bonds than single covalent bond can withstand, and that’s without considering quadrupole interactions. This has interesting consequence: as long as you can make sure that at least some of the time neighboring strands have these 30 or more hydrogen bonds between them, under tension they don’t slip - failure mode shifts. This means that kevlar tolerates some breaks, a very important property in a world where everything breaks. There’s still benefit to strands longer than that - it allows for increased tensile strength, to a degree - but it’s these weaker hydrogen bonds that make kevlar what it is, an interaction that EY dismisses outright as some superfluous bullshit not worthy of attention.
Exactly the same thing applies to peptide beta-sheets and collagen, keratin and such, which have similar hydrogen bonding pattern but this time after 3 strands it wraps over, making 3-strand helix. As it happens, alpha-aminoacid based peptides pack pretty much as many amide bonds per chain length as humanely possible, so these hydrogen-bonds-collevtively-are-stronger-than-strand segments are even shorter than in kevlar. These are actual structural proteins that are part of the reason that cells hold shape. Same goes for cellulose, chitin and to some degree lignin. The above is also the reason why i think that this bond counting metric is pretty useless, and that’s before ionic and vdW interactions swamp the entire picture. You can arrive at something more useful by counting bond energies by type, but this still makes sense only in context, because you might break single covalent bond vs bunch of hydrogen like above, so shape is also important. Even this wouldn’t be massively useful, but that could be better. But i digress
Nanomachines, son
EY makes a big point of how living thingies lack wheels, and his imaginary nanomachines have wheels, which makes them superior (yes i know about ATP synthase, this is not the point). I’m not convinced on how adding wheels on perfectly wheelless bacteria would improve them in any way, especially when what you need is something else. Square-cube law is binding here and in full effect - noncompliance is punished by inefficiency, and energy is something that will be very useful. There are already strings, levers, shuttles, springs, elastic membranes, one-way and two-way pumps and valves, some gated by external signal, and even a tiny conveyor belt at this scale. We don’t need no wheels where we’re going
I’m not sure how this entire thing would maintain integrity and remain isolated from external environment. Actual biology uses for this lipid bilayer, which can’t be easily damaged in some ways because it’s literally liquid, what’s more it can absorb some insults, it can conform to surface, it can replace its components at will - all you need to do is to overcome these weak interactions (hydrophobic in this case) and you can pull out some protein, shred it into aminoacids, and make it anew. You can’t do any of this with stiff, crosslinked sheets of graphane (or something)
Lipid bilayer has a very important function that even a small break kills catastrophically - it stores energy by way of electrical and chemical gradient across it. When it breaks, you’re proper fucked - fortunately, these weak interactions allow for membrane to reform where it was punctured. This fails badly when membrane is stiff for obvious reasons. Membrane also needs to be thick: we’re talking about some 150mV across some 5nm of membrane of a living bacterial cell, which in already existing biological systems means 30kV/mm, and can withstand much more. What was that supposed benefit? Higher energy/power density? You would need to think really hard about making materials that can withstand this and higher fields. Note: real life example already uses pretty much the best material for the job
Everything breaks 2
In real life everything breaks. Proteins misfold, denature, get pieces chopped off by hydroxyl radical. This is not something you can magic away: reactive oxygen species form wherever there is oxygen and some heavy metals in biological systems, and more generally everywhere where there’s water and ultraviolet at the same time. Nature has a solution: just shred everything exposed periodically, at random. This way, as long as the cell is alive, no critical protein lives long enough to accumulate too much damage. Fortunately, proteins aren’t disassembled into atoms - building blocks can be put back with some limited effort into new intact copies (
1 ATP per residue, + some extras for folding, transport, unfolding DNA, let’s say conservatively 1.5 ATP per residueactually looked it up, it’s 4.2 ATP per residue. point still stands).This rather economic recycling allows a living cell to absorb damage that would be catastrophic when you just assume that everything works forever just as you imagined. I don’t have a guess how much more energy would be expended in reassembly of diamondoids, @titotal@awful.systems might have an estimate, but i guess it’s some 1-2 orders of magnitude more. Disassembling and assembling everything at random would be most likely prohibitively expensive in terms of energy, and detecting fault could be very easily hard to impossible. (There are some pathways that are responsible for shredding misfolded proteins, again, you can’t do this with stiff things)
Assorted shit
Nowhere is explained where energy comes from, this is probably the biggest issue in all of this. From what i understand, all this assembly by manipulator magic also only ever happens in high vacuum, at cryogenic temperatures (?) at tip of AFM, and on top of all of that entire surface to be worked on has to be uncapped (ie covered in radicals). This is not exactly a condition directly transferable to something that has to survive in air or even worse, in water. Nowhere is also explained where, or how, information about structure is stored.
Somebody in the comments made a point about how they’re sure that you can make something like a protein but held entirely by covalent bonds - while possible in principle, i find notion of such thing absurd and detrimental to fitness - you can’t easily make or hydrolyse such densely, permanently crosslinked protein in usual ways, it would require special proteases. Even some cyclic peptides resist digestion, that’s because normal proteases turn proteins into unfolded string of aminoacids first and chop it one by one. You can’t do this when there’s no end. This gets much worse when it’s an entire 3d mesh of mess that you can’t even move, and there’s already an actual biological problem with this - it’s linked to nonspecific reaction of glucose with proteins, most commonly, tying permanently lysine and arginine side chains. These are quite appropriately abbreviated as AGEs https://en.wikipedia.org/wiki/Advanced_glycation_end-product
Why don't organisms make diamond bones? Are they stupid?
Well, partially, IIUC, because calcium atoms are heavier than carbon atoms. So even if per-bond the ionic forces are strong, some of that is lost in the price you pay for including heavier atoms whose nuclei have more protons that are able to exert the stronger electrical forces making up that stronger bond.
This is so fucking wrong i had to quote this. I don’t even know where to begin, calcium atoms have charge +2 in hydroxyapatite and pretty much in any other non-pathological edge case. The bigger (heavier) you make an atom, the less surface charge becomes, this is a bad thing if you want strong ionic bonds
But then, why don’t diamond bones exist already? Not just for the added strength; why make the organism look for calcium and phosphorus instead of just carbon?
Because it’s already here and making diamond bones is both pointless and prohibitively energy expensive. I guarantee there would be enzymes running on lanthanides, were these more common in, say, seawater
Ionic bond is not really a bond in some senses of this word. It’s better to say ionic interaction - bond in some meanings has defined shape, that is there is a geometry that obtains minimum energy. This works in salt crystal, where any disturbance of ion position has to happen along sharp energy gradients. It doesn’t really mean anything in proteins, where charges are fuzzier, further apart, and there’s water around blanketing every charge and shielding it from others. Here, there might be some things that are attached directly to hydroxyapatite crystal by ionic interactions, I expect some phosphorylated proteins, but don’t quote me on that
I don’t even think we have much of a reason to believe that it’d be physically (rather than informationally) difficult to have a set of enzymes that synthesize diamond.
Yeah, I’m pretty sure that would be rather pretty fucking hard, especially when you don’t have things like palladium or platinum. Making C-C bonds in controlled way is hard enough, making them in regular lattice without any functional groups nearby makes it significantly worse
Nobody should think about anymore, none of that nanomachine drivel makes sense. When in doubt, don’t
Thanks, I love these answers! I’ll drop a DM on matrix for further questions.
This rather economic recycling allows a living cell to absorb damage that would be catastrophic when you just assume that everything works forever just as you imagined. I don’t have a guess how much more energy would be expended in reassembly of diamondoids, @titotal@awful.systems might have an estimate, but i guess it’s some 1-2 orders of magnitude more
The DMS researchers were estimating something on the order of 5 eV for mechanically dropping a single pair of Carbon atoms onto the surface of diamond. I’m not sure how to directly compare this to the biological case.
so we’re looking at something in the order of 2x more energy for pair of carbons than for putting single aminoacid in protein, and aminoacids are much larger. (some 4.2x70kJ/mol per ATP -> AMP + 2Pi), and that’s including everything around protein synthesis except aminoacid synthesis, compared to just deposition of carbons (that’s without picking them up?), and it’s when you have clean prepared surface, in real life this also takes some considerable energy
re: energy requirements, there’s a bit about dissipative systems here that i never could get fully because it’s some harder thermodynamics that i’ve been used to. pretty neat stuff https://en.wikipedia.org/wiki/Dissipative_system
The paragraph about gullibility resonates strongly with me. One of the first things in organic chemistry (a course I repeatedly failed) is that carbon doesn’t behave like the ions which we normally manipulate in undergraduate chemistry laboratories. Instead, carbon is like a Lego brick or K’Nex connector, with four ports which clip together in a variety of configurations. This is used to explain many quirks of biology, like why diamond or nanotubes can’t be easily produced by enzymatic processes; as you explain, carbon’s bonding process makes it very difficult to put into place atomically, and instead we need some sort of external force like immense pressure or heat to reconfigure large masses of carbon into carbon-only structures.
deleted by creator
I suppose when talking about science to a popular audience it can be hard not to make generalizations and oversimplifications and if it’s done poorly that oversimplification can cross over into plain old inaccuracy (if I were to be charitable to Yud I would say that this is what happened here).
To wit: even the “K’nex connector with 4 ports” model of carbon doesn’t really explain the bonding of aromatic molecules like benzene or carbon nanotubes; I’ve likewise seen people confidently make the generalization “noble gases don’t react”, apparently unaware of the existence of noble gas compounds.
Thanks for all the effort, also that you post these on all the various LW-sphere related places. Interesting to see the various places react.
E: 18 hours later, I notice that on LW your 38 vote score went down to 30 while the amount of votes increased from 17 to 70, and on EA it also dropped from a higher positive (forgot how high) to a sad 9.
Big Yud himself responded:
edit: there’s lesswrong thread too: https://www.lesswrong.com/posts/8viKzSrYhb6EFk6wg/why-yudkowsky-is-wrong-about-covalently-bonded-equivalents
“If you take only the statements where I was vague instead of the ones where I was explicitly wrong and interpret my words in the way that I am now telling you to, you will see that I am right.”
Yudkowsky:
Talking to the general public is hard.
Multiple commenters on FanFiction.net replying to chapter 23 of HPMOR: Genetics don’t work that way. If magic were recessive, then wizard parents would always have wizard kids and there would be no such thing as squibs. Look, I drew the Punnett square…
Don’t patronize fans, Yud.
“I’m LessWrong than you’re implying!!!”
I must have missed the class in material physics where they explained that all material has a generic “strength” that determine which material can cut which. Is it perhaps abbreviated STR?
Only someone with high INT can discover this brilliant theory. As luck would have it, they have high CHR too!
Unfortunately such characters tend to dump stat WIS.
He never played dwarf fortress confirmed, else he would be talking about shearing, compression, tearing, impact and whatever else values DF uses for materials.
The so called “experts” say that spider silk is stronger than steel, but steel beams can hold up bridges while I can break a spider web with my little finger. Looks like the “experts” are wrong and spider silk isn’t very strong after all - probably because it’s made of proteins held together by weak van der Waals forces instead of covalent bonds.
@GorillasAreForEating @mountainriver
Yes but if you had a five ton, meter wide strand of spider silk…
I reply: Because the strength of the material is determined by its weakest link, not its strongest link. A structure of steel beams held together at the vertices by Scotch tape (and lacking other clever arrangements of mechanical advantage) has the strength of Scotch tape rather than the strength of steel.
This is sub-childishly false and he opens with it. Unbelievable.
He also writes: “The entire human body, faced with a strong impact like being gored by a rhinocerous horn, will fail at its weakest point, not its strongest point.”
If a rhino comes at Yud, he can use his mighty cranium, which is not his weakest spot, to defend his weak meat parts. Since the rhino horn only impacts his head and not his weak points, his body can not fail, and thus he lives.
Reminds me of Cyrano de Bergerac’s Travel to the Sun, where the protagonist encounters a thin chain carrying a great load. Since all links of the chain were equally strong, it couldn’t break as chains always break in there weakest link. De Bergerac had the excuse of writing his sci fi in the 17th century (he also features some pre-Newtonian physics), Yud lacks such an excuse.
There are too many comments in here going for the stringy lean detail and not pointing out magnificent conceptual errors like this
Quoth Yud:
I’m sort of skeptical that you could write something that works as science communication for a general audience, though lord knows I’m not necessarily succeeding either.
All the faux modesty of Tommy Tallarico saying “my mother is very proud”.
The key valid ideas to be communicated are [made-up sci-fi bullshit about nanobots]
Bone is weaker than diamond, then, because… why?
Well, partially, IIUC, because calcium atoms are heavier than carbon atoms. So even if per-bond the ionic forces are strong, some of that is lost in the price you pay for including heavier atoms whose nuclei have more protons that are able to exert the stronger electrical forces making up that stronger bond.
i don’t even know where to begin. stay in school, kids
Guy with 4 wiki pages open, determined to win an argument, even if it means stacking shit until the other person stops responding.
this entire response reads like “diamond is the hardest metal” copypasta
“yeah but no also :words:”
deleted by creator
If I were inclined to be charitable, I’d wonder who he was talking to that honestly thought in terms of “elan vital” and “game balance”. Like, take the question “Why is flesh soft and diamond hard?”, and ask somebody without a science background. I’d guess that the typical response would go something like, “I’unno, it just is.” Or, “I’ve never thought about it, why?” Or, “Heh heh heh, you said hard”. I suspect that Yud is making up a layperson of his own invention, a mythical audience upon whom his Educate spell is perfectly effective. He refuses to admit any challenge to his mental model of how the process of explanation works.
It’s a technique he uses to get you, the reader, to understand that you aren’t the person who thinks in terms of elan vital.
In one of his essays on quantum phenomena and personal identity he does it with time. He explains something like if you think time in the universe works in the sense of clock time, then you just don’t have a clue about physical reality, so when he gets to his next point it stands in contrast to the straw layman. But his readers are obviously already the sort of people who do know that, because they’re nominally smart, education-enthusiastic western(ised) nerds, even if they understand next to nothing about how this works out in real physical theory.
So the strawman doesn’t just create a favourable contrast for Yudkowsky’s argument, it constructs them as smart and different from lay people - it isn’t a one-shot effect, it builds as he starts small and piles on increasingly esoteric speculations (even if this is the first “mind = blown” blog post they’ve ever read from this weird guy).
deleted by creator
I’ve been saying this more often lately, but LessWrong gets its readers in, by and large, at the absolute bottom rung of intellectual thought, they don’t know anything else
You have to interpret somebody getting into LessWrong as just graduating from Cracked or Newgrounds in the mid-2000s
LessWrongers don’t have a sense of a “vaguely plausible normie” that is calibrated in a grounded way (by trying to teach the Intro for Non-Majors courses, trying to explain to relatives and high-school friends what you do for a living, etc.). Instead, they have a concept constructed to set them apart and above. The normie is the ideal rube, the complete inverse of the sophisticate that the LessWronger aspires to be or believes themselves to have become.
Garynita musgyraxia shrooms. Very rare, only grows on moldy first edition dnd books.
deleted by creator
trumpian
Okay that’s so much word vomit, and I know next to nothing about biology and medicine so I have to ask: is any of this actually relevant to pandemics, virulence, lethality or whatever was his initial point?
His argument, as I understand it, is that he knew about the covalent bonds between proteins but didn’t mention them because he was simplifying things for a lay audience, and that those covalent bonds don’t matter because they aren’t the “load bearing” elements in flesh.
There are two problems I see
-
His earlier statements suggest he actually had no knowledge of that whatsoever
-
I think his revised explanation is still wrong, because the extracellular matrix that holds cells together and connective tissue are composed largely of proteins that have these covalent crosslinks and rely on them for strength. When you tear a ligment it’s not just van der waals and hydrogen bonds being broken, those alone would be far too weak.
What I mean specifically is, he wrote:
The nanomachinery builds diamondoid bacteria, that replicate with solar power and atmospheric CHON, maybe aggregate into some miniature rockets or jets so they can ride the jetstream to spread across the Earth’s atmosphere, get into human bloodstreams and hide, strike on a timer.
Would “diamondoid bacteria” be inherently, significantly better at killing us? Or wait is he imagining the bacteria literally slashing at us???
From what I could understand is that he talks about diamondoid (and these other things) just because he has read one book about the subject. ‘Nanosystems’ by Drexler apparantly. (Never read it, can’t say anything about it).
I’m not sure Yud is really engaging with what is being said vs just going on and on about how AGI can kill us all via nanomachines (son), because handwave theory something.
It’s like he heard the phrase “flesh-eating bacteria” and decided they would be more scarier if they had tiny knives and forks.
The worst part is when they start to season you with salt and pepper…
-
It was in high school that I last learned about the different types of bonds. This one article actually gave me a better education than chemistry class, if I’m going to be very honest. (Though to be fair to my chemistry class, I probably wouldn’t have the foundational knowledge to understand what was going on otherwise, so I’ll give you both credit)
Quoth Yud:
Algae are tiny microns-wide solar-powered fully self-replicating factories that run on general assemblers, “ribosomes”, that can replicate most other products of biology given digital instructions.
Ribosomes … make proteins.
and not even finished proteins
When, arguing with people like yudkowsky, you can never decisively ‘win’ or change his mind, because he and other doomers can quickly retreat to the classic hole: “You can’t prove X is impossible!! Nature isn’t already perfectly optimal!!!” Searching for some kind of “hard limit” on how nature or technology can evolve will always end up empty handed. Lots of really awful things are possible. (Lots of super fascinating things are also possible.) Searching for some singular hard reason why nature as it is, is totally safe from future threats or change will always end up empty handed.
Capability, is not interesting. Capability, is not the real test. Economics, is the real master of it. And specifically, the open system economics of the entire environment in which something is embedded. It’s why the Voyager, a technology planned, built, and launched with 80 year old techniques and knowledge is SOTA for space exploration and contribution to science, and Starship is still just a huge dark hole for money and talent.
if I want to understand historical biology, I do not go looking for the alien intelligence and engineering capability that built it, I look for the environmental forces that contributed to, and eventually supported the homeostasis of, it.